UMSOP’s Pearson, Kane, and Jones Labs Publish Article in Nature Communications
New study sheds light on how disease progression and biomolecular corona affect nanoparticle-mediated immune activation.
By Pam Carder
March 13, 2025
Researchers at the University of Maryland School of Pharmacy (UMSOP) have published an article in Nature Communications focused on characterizing the interaction between nanoparticles and the immune system and the factors that influence therapeutic outcomes.
The collaborative, multidisciplinary effort sheds new light on the complexities of nanoparticle-based therapies and offers a fresh perspective on why so many nanomedicines fail during clinical trials. It was spearheaded by first author Jacob Shaw, recent PhD graduate with the Pearson Lab at UMSOP, and Ryan Pearson, PhD, associate professor of pharmaceutical sciences (PSC) and director of the School of Pharmacy’s Bio- and Nano-Technology Center (BNTC).
“Nanoparticles have great potential in improving the delivery of therapeutic modalities, but their clinical translation has been limited by interpatient variability,” says Shaw. “Our results highlight the need to develop more personalized therapeutic regimens to prevent unintended immune-reactions.”
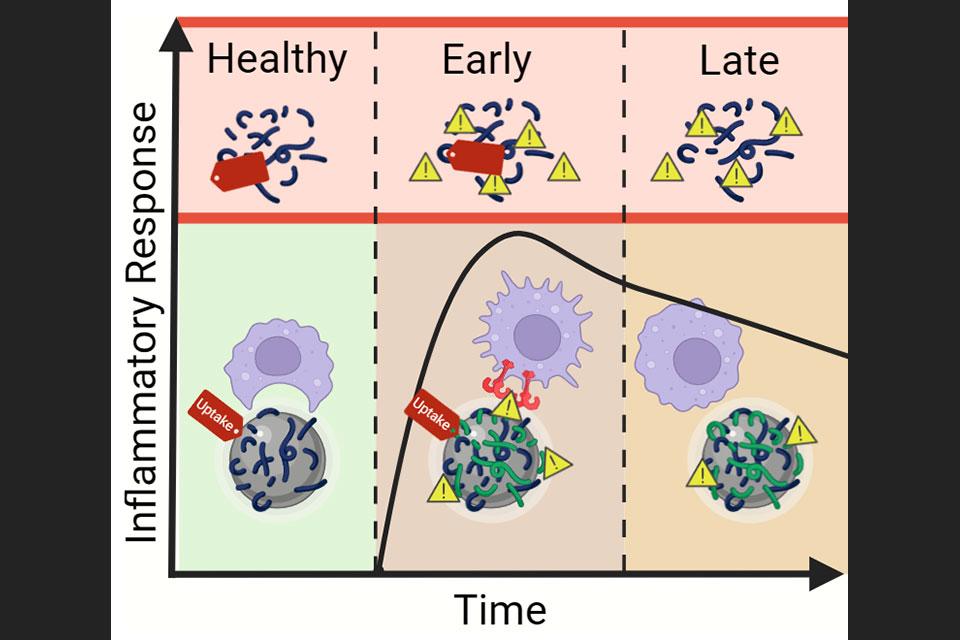
The research team hypothesized that the stage of a disease—early, mid, or late—can influence the composition of biomolecules in a patient’s blood. This, in turn, affects how nanoparticles accumulate and interact with immune cells, ultimately impacting the therapeutic efficacy of nanoparticle-based drugs.
“Our two-and-a-half-year study tackles a longstanding dilemma in the field of drug delivery regarding why patients with the same disease often experience vastly different therapeutic responses,” says Pearson. “Our research addresses a key challenge in nanomedicine, which is understanding the variability between patient responses and the failure of targeted therapies, such as those aimed at treating cancer or inflammation.”
The study, funded by the NIH National Institute of General Medical Sciences, focused on a phenomenon known as the biomolecular corona. When nanoparticles are introduced into a biological system, biomolecules from the surrounding fluid (like blood) attach to their surfaces, changing the particles’ identity. This “biological identity” can alter how nanoparticles interact with immune cells and influence the immune response. These findings highlight a critical gap in existing research—most clinical trials have not considered how disease stage and the biomolecular corona affect the efficacy of immunotherapies and nanoparticle drugs.
The study team included graduate students and postdoctoral fellows from Pearson’s lab, as well as researchers from the labs of Maureen Kane, PhD, professor of PSC and executive director of the School of Pharmacy’s Mass Spectrometry Center, and Jace Jones, PhD, associate professor of PSC and associate director of the Mass Spectrometry Center.
“It is always exciting to collaborate with departmental colleagues and see these collaborations result in a high-quality publication, as was the case here,” says Jones. “The publication was a result of Dr. Pearson’s vision and his lab’s effort to integrate multiple disciplines in a coherent and meaningful way.”
Nicholas Caprio, PharmD ’24, an early oncology clinical science fellow at AstraZeneca, contributed significantly to the project as a Doctor of Pharmacy student at the School of Pharmacy, dedicating multiple years and summers to the research, earning him second authorship.
“We’re trying to change the way we think about nanoparticle therapies,” says Caprio. “It’s not just about having a drug that works in a lab; we need to understand how it will work in the real world, where patients have varying health conditions that could impact their response.”
The study highlights the need for more thorough and individualized approaches in clinical trials and drug development. The team hopes to work on developing predictive models that integrate patient data, biomaterial characteristics, and comprehensive testing to optimize the therapeutic effectiveness of nanoparticles. This approach could potentially lead to more personalized treatment options and increase the success rate of nanomedicines in clinical trials.
“We couldn’t have done this work without a collaborative approach between multiple labs at the School of Pharmacy,” says Pearson. “By bringing together experts from the Jones lab, who focused on lipid analysis, and the Kane lab, which examined proteins and metabolites, we were able to develop a comprehensive understanding of how disease states influence the biomolecular corona. This collaborative effort allowed us to explore questions that would have been impossible to answer in a single lab setting. We hope this study encourages further interdisciplinary work to enhance the predictability and success of drug delivery systems.”
Authors of the study are Jacob R. Shaw, Nicholas Caprio, Nhu Truong, Mehari Weldemariam, Anh Tran, Nageswara Pilli, Sarnima Pandey, Jace W. Jones, Maureen A. Kane, and Ryan M. Pearson.
Read the full publication, “Inflammatory disease progression shapes nanoparticle biomolecular corona-mediated immune activation profiles” in Nature Communications.


