Study Aims to Develop Faster Techniques for Identifying Bacterial Infection
NIH-funded study focuses on novel means of bacterial identification that could provide hospitals with a more rapid and accurate assessment of infections.
By Becky Ceraul
April 2, 2015
Faculty members from the University of Maryland Schools of Pharmacy and Dentistry have received a $1.1 million grant from the National Institute of General Medical Services at the National Institutes of Health to create a bacterial library using mass spectrometry technology. The library, being developed by David Goodlett, PhD, the Isaac E. Emerson Chair of Pharmaceutical Sciences at the School of Pharmacy and Robert Ernst, PhD, an associate professor in the Department of Microbial Pathogenesis at the School of Dentistry, will allow physicians to more quickly and accurately identify a patient’s infection, leading to more accurate and quicker treatment.
“Rapid and accurate pathogen detection and identification is sorely needed in hospitals and clinics to allow physicians to react and respond appropriately to potentially life threatening infections,” says Goodlett, who is also director of the School of Pharmacy’s Mass Spectrometry Center. “So often we hear about patients who were given antibiotics for an infection that wasn’t properly diagnosed because of limitations with the diagnostic process. Antibiotics can be damaging to the kidneys and the bone and can lead to issues with antibiotic resistance, so we want to improve upon existing diagnostic tests in order to improve treatment.”
The current methods for diagnosing bacterial infections that are approved by the Food and Drug Administration include biological culture, nucleic acid amplification, ribosomal protein sequence characterization, and genome sequencing. “Collectively, these methods are slow, require amplification of clinically-obtained material, and are often significantly expensive and burdensome for diagnostic laboratory staff,” says Ernst. “Our study aims to create a library of chemically barcoded bacteria that can be checked to determine which infection a patient has. This will ultimately be faster, cheaper, and more accurate.”
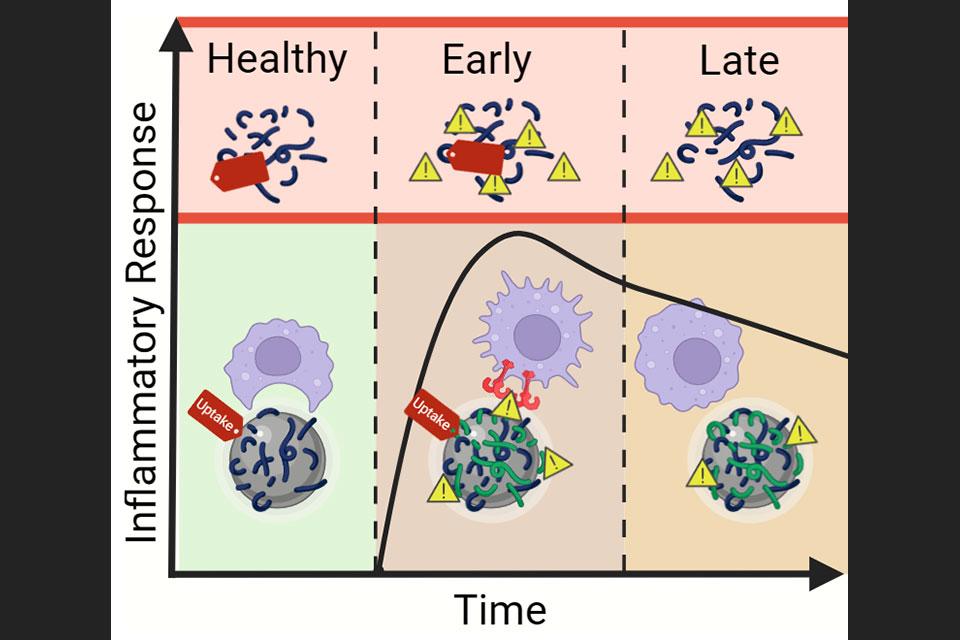
In order to improve the rapid and accurate diagnosis of bacterial infections, Goodlett and Ernst will use a mass spectrometry technique called matrix-assisted laser desorption ionization, or MALDI. “Through this study, we will develop, refine, and use ultra-small scale purification methodologies for the extraction of essential, high abundance lipids from Gram-positive and –negative bacteria, as well as fungi,” says Goodlett. “These lipids are found in all membranes of microbes and are a highly diverse set of molecules. This diversity forms the basis of our hypothesis that essential bacterial and fungal lipids constitute a chemical barcode that can be used to identify pathogens by mass spectrometry profiling.”
Goodlett’s and Ernst’s preliminary data show that these lipid structures are unique and can be used as novel chemical barcodes for the identification of bacterial and fungal infections and resistance patterns to a subset of antibiotic and antimicrobial peptides. “Lipids will be analyzed by mass spectrometry with the results used to generate a mass spectral signature library of lipid ‘fingerprints’ from a wide variety of clinically-relevant pathogens,” says Ernst. “The combined analysis of the protein and the lipid will provide a greater than 99 percent accuracy level in identifying bacteria from a variety of human samples.”
“The methods Drs. Goodlett and Ernst are developing will change how infections are diagnosed in clinics, dropping the time it takes from days to hours,” says Andrew Coop, PhD, professor and chair of the Department of Pharmaceutical Sciences at the School of Pharmacy. “Their collective expertise in infectious diseases and mass spectrometry, combined with the extensive resources of the Mass Spectrometry Center, will translate into improvements in patient care.”
In order to translate the technology fueling the bacterial library concept into commercially available products that benefit patients, Goodlett and Ernst are working with the University of Maryland, Baltimore’s (UMB) Office of Technology Transfer to identify companies interested in developing the needed software for the project and to assist with the development of assays to detect the bacteria.
“Our office is very excited about this technology,” said Phil Robilotto, assistant vice president of the Office of Technology Transfer at UMB and chief commercialization officer for UM Ventures. “Drs. Goodlett and Ernst are developing a truly novel point-of-care pathogen identification product. The BacLib detection system’s speed and ability to utilize clinical samples without culture makes the system extremely promising from both a patient care and commercial perspective.”